Introduction
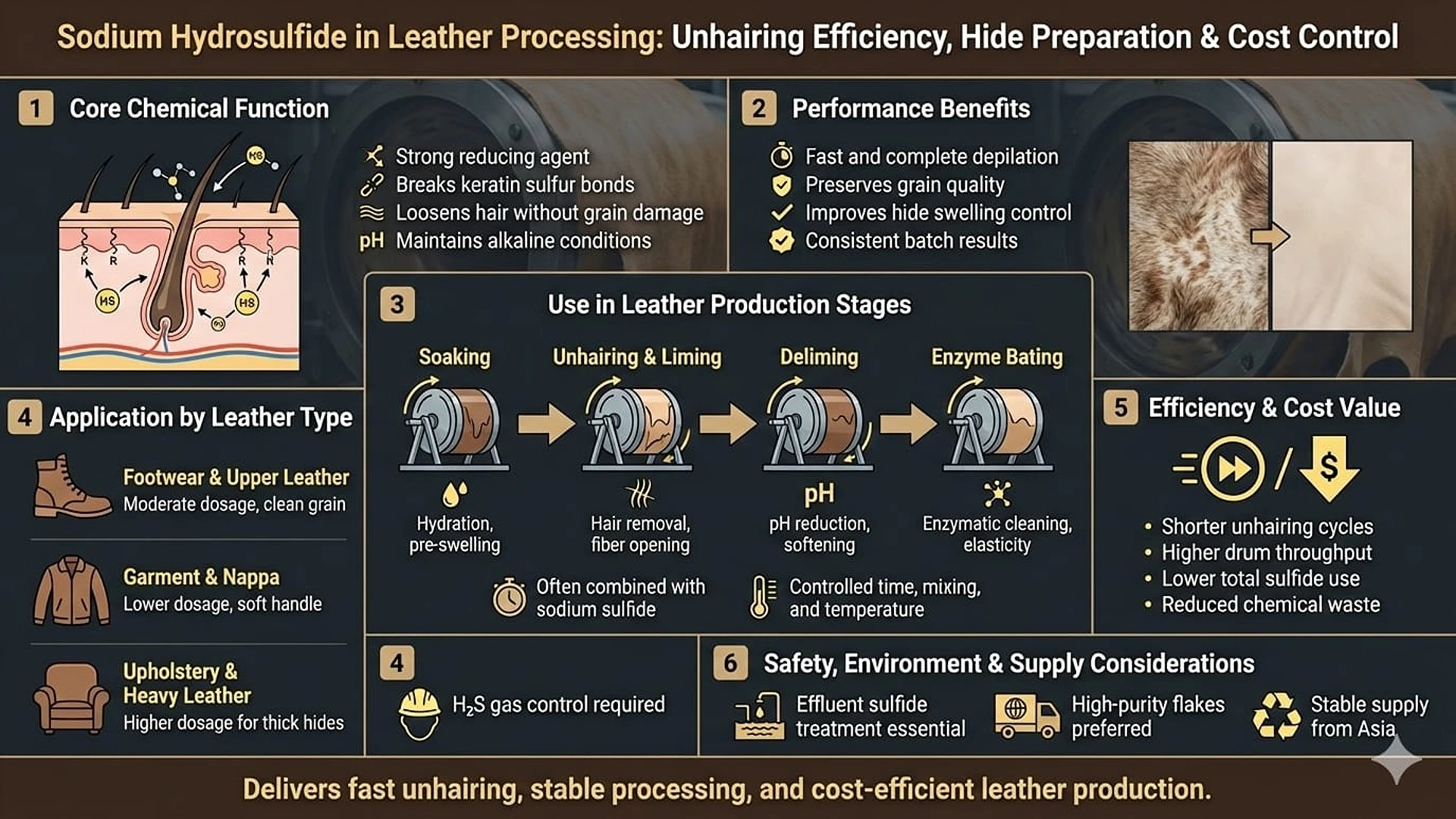
Sodium hydrosulfide is a key chemical used in leather manufacturing, mainly during early hide preparation. It plays an important role in removing hair and opening up the hide structure before tanning. Leather producers rely on sodium hydrosulfide because it works fast, delivers consistent results, and helps control overall production costs in beamhouse operations.
Chemical Functions in Leather Processing
Sodium hydrosulfide works mainly as a reducing agent. It breaks the sulfur bonds found in keratin, the main protein in hair and the outer skin layer. Once these bonds are broken, hair loosens easily and can be removed without damaging the hide surface.
At the same time, sodium hydrosulfide keeps the processing bath strongly alkaline. This alkaline condition helps swell the hide, making it easier for chemicals to enter evenly. Compared with lime-only systems, sodium hydrosulfide removes hair more gently and preserves the grain layer, which is important for high-quality leather.
Its reducing action also limits damage to collagen fibers. This helps maintain strength and smoothness, which are critical for upper leather used in footwear, upholstery, and automotive interiors.
Use in Key Leather Production Stages
Sodium hydrosulfide is mainly used during the unhairing and liming stage. After soaking, hides are treated with sodium hydrosulfide solutions for several hours. This step removes hair and prepares the hide for further processing.
For thicker hides such as cattle hides, sodium hydrosulfide is often used together with sodium sulfide. This combination improves efficiency and allows lower total chemical use. The process is carefully controlled with mixing and temperature adjustment to ensure even results.
Later, during deliming, remaining alkalinity from sodium hydrosulfide helps neutralize lime. This prepares the hide for enzyme bating, which removes unwanted proteins and improves softness. Wastewater from this stage contains sulfides and must be treated before discharge.
Differences Across Leather Segments
Different leather products require different sodium hydrosulfide levels. Footwear and upper leather need clean and even hair removal, so moderate dosages are used to protect the grain surface. Garment and nappa leathers use lower amounts to avoid excessive swelling and keep the leather soft.
Heavy leather, such as sole or upholstery leather, often uses slightly higher levels to deal with thicker hides. In these cases, longer processing times help achieve full depilation without over-processing.
Outside the leather industry, sodium hydrosulfide is used in mining, paper, and textile applications, but leather production requires higher purity grades. Impurities can cause stains or uneven processing, so leather tanneries demand consistent quality.
Operational Value and Efficiency
Sodium hydrosulfide improves efficiency by reducing unhairing time compared with traditional lime-sulfide methods. Faster processing increases drum productivity and allows more hides to be processed each day.
Because it dissolves easily in water, sodium hydrosulfide spreads evenly throughout the drum. This reduces chemical waste and improves control over results. Its stability in alkaline conditions also supports consistent performance from batch to batch.
From a cost perspective, sodium hydrosulfide often replaces larger amounts of sodium sulfide. This lowers overall chemical usage and helps manage costs, especially when sulfur prices fluctuate. Bulk storage and flake form also support efficient transport and long shelf life.
Safety, Environmental, and Supply Considerations
Sodium hydrosulfide releases hydrogen sulfide gas under certain conditions, so proper ventilation and safety systems are required. Tanneries must follow strict handling rules to protect workers.
Effluent treatment is essential. Most facilities oxidize sulfides or use iron salts to remove them before discharge. This helps meet environmental limits and supports sustainable leather production.
Supply mainly comes from large chemical producers in Asia. Buyers focus on purity, low iron content, and consistent flake size. Long-term supply agreements help reduce price volatility and ensure steady availability.
Conclusion
Sodium hydrosulfide remains a core chemical in leather processing due to its strong depilatory action, fast performance, and reliable results. When used correctly, it improves leather quality, shortens production cycles, and supports cost control. With proper safety measures and wastewater treatment, sodium hydrosulfide continues to play a vital role in efficient and competitive leather manufacturing.
Leave a Comment